
60 aniversary of an organization with a young spirit, with a humanistic profile and an innovative heart.

Inauguration of the new Logistics Center in Camino Carrasco 5119, Montevideo.

The construction of the new Logistics Center starts on Camino Carrasco 5119, Montevideo.

Laboratorio LIBRA inaugurates its new offices and begins the expansion works at the productive plant.

LIBRA acquires a new facility building located in Solferino 4096 and Avda. Italia, destined to the administrative areas, which results in a modernization of its offices and an expansion of its administrative and technical capacity.

The 1st Comparative Study on original antibiotics against LIBRA generics is carried out in Chile in 349 patients and guarantees the quality of its products (Piperazam, Cefactam e Imipen).

Laboratorio LIBRA celebrates its first half century of existence with the acquisition of a plot nearby the Carrasco International Airport for further capacity development.

Certification of GMP compliance is renewed by ANVISA. Extension of area for SUME-CEMEPA and SUME-CITOS sectors for Nutrition admixtures and Fractioning of cytostatics.

It receives the Exporting Innovation Award 2009 which is granted by The Exporters Union of Uruguay and the Uruguayan State Bank, Banco República Oriental del Uruguay. Cutting-edge pump is added to the SUME-CEMEPA area.

It devotes a new area to CEMEPA-NUTRITION and SUME-CITOS sectors for the elaboration of parenteral admixtures.

In Uruguay, 1,200 square meters are added to the Distribution sector, to be in line with the new volumes handled by Laboratorio LIBRA. In Brazil,it tranfers its Quality Control Laboratory from Vitoria to Porto Alegre and enlarges its facilities. Consolidation in the Central American market. It sold more than 100 products abroad.

A pharmaceutical bioequivalence certification program of its products in Brazil is started. Certification of exchangeability for sterile antibiotics and oncology drugs, acknowlwdged by ANVISA, are obtained. Laboratorio LIBRA products are exported to Vietnam for the first time, opening the market of the Asian continent.

Laboratorio LIBRA Uruguay reforms its microbiology sector, following the same segregation guidelines used in production.

LIBRA opens its Quality Control Laboratory in Vitoria, Brazil.

Renovation and extension of manufacturing, conditioning and distribution plant in Uruguay.

In its facilities, CEMEPA-CITOS started for the reconstitution of Hemato-oncology drugs. Major exports are made to Cuba.

Laboratorio LIBRA products are exported to new markets outside MERCOSUR, such as Peru and Ecuador.

It opens its offices in Chile.
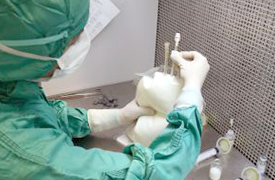
In its facilities, CEMEPA, the Parenteral Admixture Center, started for the preparation of parenteral nutrition.

LIBRA is the first Uruguayan laboratory to receive the GMP certificate granted by the health authorities of ANVISA Brazil, a guarantee that is renewed periodically.

Moves its plant to the Arroyo Grande St. location and builds a facility for segregated products for injection (cephalosporins and penicillins), placing itself as a leader of the most rigorous demands.

Opens offices in Brazil.

Starts operations in Paraguay.

In accordance with other Uruguayan companies and government support, the quality control laboratory with the most advanced technology of the time is inaugurated.

Laboratorio LIBRA starts to sell products from their own production.

Founded in 1961 by the Pharmaceutical chemistry Carlos Scherschener Kohn
